Abstract
Introduction: Daratumumab is a human IgG1k monoclonal antibody that binds with high affinity to a unique epitope on CD38.Daratumamab in combination with bortezomib or lenalidomide and dexamethasone has been shown to be effective in heavily pre-treated patients with relapsed or refractory multiple myeloma. The aim of this study was to analyze the clinical outcomes of the combination of Daratumamab, pomalidomide and dexamethasone in relapsed/refractory myeloma patients at our institution.
Methodology: We treated 20 patients with relapsed and relapsed refractory multiple myeloma at the University of Kansas Health System between November 2015 and July 2017, with Daratumamab, pomalidomide and dexamethasone (DPd) combination. Descriptive analyses were performed on available data for patient characteristics, disease course, and outcomes. Responses were evaluated using the IMWG criteria.
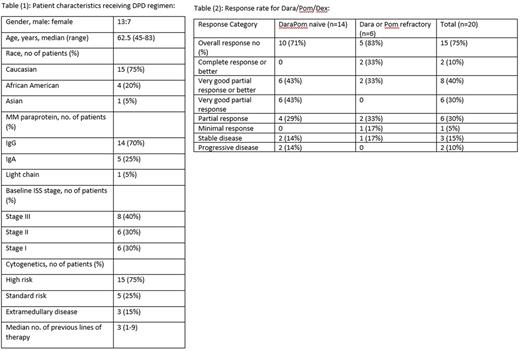
Results: Among the 20 patients included in the analysis, the median age at the time of receiving the DPd is was 62.5 years (range 45-83). Fourteen patients (70%) had IgG isotype, 8 patients (40 %) had ISS stage III disease at diagnosis, 15 patients (75%) had high risk cytogenetics, and 3 patients (15%) had extramedullary disease. Patients characterized are summarized in table 1. Median number of DPd cycles received was 5 (2-20) cycles, median duration of treatment was 5 months (2-19) months. The median number of previous lines of therapy was 3 (1-9). Fourteen patients (70 %) were bortezomib refractory, 15 patients (75%) were refractory to lenalidomide, 12 patients (60%) were refractory to carfilzomib, six patients (30%) were refractory to either pomalidomide or daratumumab, 5 patients (25%) were refractory to pomalidomide, 4 patients (20%) were refractory to daratumumab, 3 patients (15%) were double refractory to daratumumab and pomalidomide. Seventeen patients (85%) had received ASCT prior to DPd, 3 patients (15%) received second ASCT, 1 patient (5%) received a third ASCT prior to DPd. The ORR for all patients was 15 patients (75 %), however the response rate for those who were refractory to either Pomalidomide or daratumumab was 6 patients (83%), the response rates and disease characteristics are summarized in table 2.
Conclusions: Our analysis shows an impressive overall response rate in those with Protesome inhibitor and Immunomodulator refractory in relapsed and Relapsed/refractory myeloma, however an impressive response rate of 83% was noticed with those patients who were refractory to either pomaliomide or daratumumab suggesting the benefits of considering the combination of the DPd regimen of treatment even for this group of patient's complex patient population.
Ganguly: Amgen: Other: Advisory Board; Seattle Genetics: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal